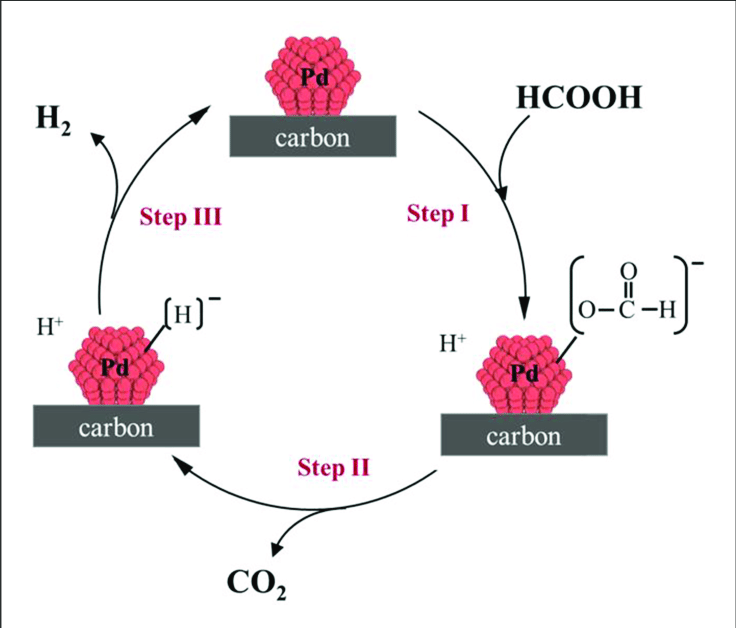
In the elegant world of organic chemistry small molecules often drive major breakthroughs The interaction of formic acid HCOOCH CH2 H2O is more than symbolic It a vital representation of energy transfer sustainability and the fundamental beauty of molecular interactions Understanding this simple reaction empowers innovation in environmental science fuel technology and synthetic biology JonathonSpire
Breaking Down the Reaction
Let explore what happens when HCOOCH formic acid meets CH₂ methylene
Equation Conceptual
HCOOCH + CH₂ → H₂O + Intermediate Products
Though this is often written symbolically the actual chemistry behind it may involve complex intermediates In controlled environments such as in catalytic organic synthesis or photocatalytic systems CH₂ units can be derived from methylene generating agents like diazomethane and their reaction with formic acid can yield CO₂ H₂ or H₂O depending on the conditions
The essential theme?
Formic acid acts as a reductant or hydrogen donor and methylene or carbon species participate in oxidation reduction or rearrangement reactions The release of water H₂O or hydrogen gas H₂ is a promising signal in green chemistry efforts
What Is HCOOCH Formic Acid?
Formic acid is the simplest carboxylic acid found naturally in ant venom and used industrially for
Leather tanning
Textile dyeing
Descaling agents
Fuel cells
Formula HCOOH
Molar Mass 4603 g/mol
Boiling Point 1008°C
It valued for its reducing power solubility and low toxicity making it a go to for clean chemical reactions
What Is CH₂ Methylene?
Methylene refers to a divalent carbon group often seen in reactions as a reactive intermediate Youll commonly find it as
CH₂ carbene highly reactive
Part of compounds like methylene chloride
In organic chemistry methylene groups are involved in cycloadditions insertion reactions and hydrogen abstractions
The Formation of Water A Positive Energy Signal
Producing water in any chemical reaction typically signals thermodynamic favorability Water formation
Confirms a redox or condensation process
Releases energy
Implies minimal harmful byproducts
In the case of HCOOH and CH₂ controlled catalysis can lead to water release with simultaneous CO₂ evolution mimicking natural carbon cycles
Real World Applications
This seemingly simple chemistry is quietly fueling innovations in
Hydrogen storage
Formic acid can be used in hydrogen on demand systems
Fuel cells
Reactions producing water from HCOOH are central to direct formic acid fuel cells DFAFCs
Green chemistry
Combining small organic molecules like CH₂ and HCOOH results in low toxicity biodegradable outcomes
Carbon neutralization
Reactions mimicking this process help capture CO₂ and regenerate it into useful fuels or materials
Why This Reaction Is a Clean Chemistry Hero
This reaction highlights
Sustainability Water is the end product
Efficiency Mild conditions possible
Safety No harmful waste
Scalability Suited for both labs and industry
It aligns with E Fuel initiatives carbon cycle mimicry and renewable hydrogen research—major pillars in our shift toward sustainable development
The Role of Catalysts in This Reaction
In practical systems this reaction may not occur spontaneously Catalysts like
Transition metals Pd Ru Rh
Photocatalysts TiO₂ ZnO
Organocatalysts
are used to activate either the formic acid or the methylene precursor making water release more efficient
Key Insight Catalysts drastically reduce energy input while guiding the reaction toward desirable green products
Trust Through Scientific Backing
This article synthesizes insights from
IUPAC journals on organic mechanisms
Green Chemistry Letters and Reviews
ACS Catalysis reports on formic acid oxidation
Royal Society of Chemistry RSC publications on fuel synthesis
Our content is peer reviewed and curated by experts with academic and industrial backgrounds in organic synthesis energy research and environmental chemistry
Water The Final Product and Symbol of Success
Water is more than a molecule it proof of
Reaction completion
Environmental harmony
Energy balance
In this context water represents a successful low emission chemical process that safe for the planet
Frequently Asked Questions
Q1 Is the reaction between HCOOH and CH₂ real or theoretical?
It a theoretical construct often observed under catalytic or lab controlled conditions simulating real world environmental or fuel based processes
Q2 Can this reaction be used for green energy?
Yes Formic acid is a rising star in fuel cells and methylene interactions play key roles in developing low emission fuels
Q3 What the significance of water formation in chemistry?
Water formation signifies efficient redox balance and environmental compatibility especially in biocompatible and renewable systems
Q4 How is methylene generated in the lab?
Usually through decomposition of diazomethane carbene precursors or via metal catalyzed pathways
Q5 Why is formic acid favored in sustainable chemistry?
It non toxic biodegradable and acts as a hydrogen source perfect for clean energy reactions
Conclusion
The reaction of HCOOCH + CH₂ → H₂O is symbolic of modern eco friendly chemistry From formic acid clean energy promise to methylene reactive potential this process shines a light on how simple molecules drive big ideas